Learn about our new Innovation Center Explore Here >
Solving Your Most Difficult Elastomer and Thermoplastic Design & Manufacturing Challenges for Over 75 Years

Why Minnesota Rubber & Plastics?
For over 75 years, we’ve helped world-class organizations solve difficult sealing and component challenges. Through our advanced materials lab and global manufacturing, we work with original equipment manufacturers to provide solutions to tough challenges through materials science and formulation, fully functional product prototyping, and operational excellence and supply chain consolidation. With an emphasis on research and development, Minnesota Rubber & Plastics empowers its technicians to design, formulate, develop, and test a wide range of highly engineered materials and parts.
Now Part of Trelleborg
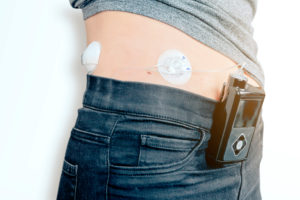
Trelleborg Sealing Solutions is one of the world’s leading developers, manufacturers and suppliers of precision seals, bearings and custom-molded polymer components. It focuses on meeting the most demanding needs of aerospace, automotive, general industrial and medical device companies, with innovative solutions.
Learn more about Trelleborg Sealing Solutions at www.trelleborg.com/seals.
Learn more about Trelleborg Medical Solutions at www.trelleborg.com/en/medical.
Our Expanded Capabilities and Global Footprint as a Part of Trelleborg Include:
- 61 Customer Solutions Centers
- 41 Manufacturing Sites
- 10 R&D Centers
- 1 Customer Innovation Center
- 5 Logistics Centers
- 3 ServicePLUS Centers
Learn about our new state-of-the-art Innovation Center.
Located adjacent to our Plymouth, MN facility.